Long Chain Alkane → Shorter Alkane + Alkene + Hydrogen
When irradiated with high energy radiation, Propane undergo cracking to form methane gas, ethene and hydrogen gas.CH3CH2CH3 (g) → CH4(g) + CH2=CH2(g)
Alkene + Air → Carbon (IV) Oxide + Water
In limited air Alkenes burn with a yellow/ luminous sooty/ smoky flame in limited air to form carbon(II) oxide and water.Alkene + Air → Carbon (II) Oxide + Water
Burning of alkenes with a yellow/ luminous sooty/ smoky flame is a confirmatory test for the presence of the C=C double bond because they have higher C:H ratio.Ethene burning in excess air:
Ethene + Air → Carbon (IV) Oxide + Water
C2H4(g) + 3O2(g)→ 2CO2(g) + 2H2O(l/g)
Ethene burning in limited air:
Ethene + Air → Carbon (II) Oxide + Water
C2H4(g) + 2O2(g)→ 2CO(g) + 2H2O(l/g)
H2C=CH2 + Br2 H2BrC-CH2Br
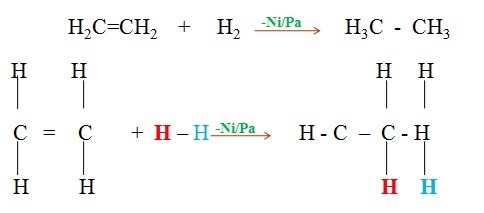
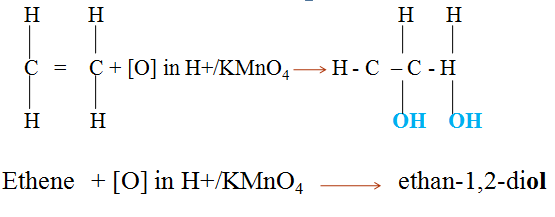
- Alkenes react with concentrated sulphuric(VI)acid at room temperature and pressure to form alkylhydrogen sulphate(VI).
- On adding water to alkylhydrogen sulphate(VI) then warming, an alkanol is formed.
Alkenes + concentrated sulphuric(VI)acid → Alkylhydrogen Sulphate(VI)
Alkylhydrogen Sulphate(VI) + Water (then warm) → Alkanol