Question
The chromatography below shows the constituents of a flower extract using an organic solvent
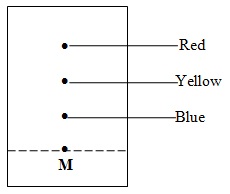
(a) (i) Name a possible organic solvent you can use for this experiment
(ii) State one property that makes the red pigment to move the furthest distance from M
(iii) Describe how one could get a sample of yellow pigment
(iv) On the diagram indicate solvent front
(b) Describe how Aluminium chloride can be separated from a mixture of aluminium chloride and sodium chloride

(a) (i) Name a possible organic solvent you can use for this experiment
(ii) State one property that makes the red pigment to move the furthest distance from M
(iii) Describe how one could get a sample of yellow pigment
(iv) On the diagram indicate solvent front
(b) Describe how Aluminium chloride can be separated from a mixture of aluminium chloride and sodium chloride
Answer
a)i)Ethanol, acetone (any organic solvent)
ii) Its most soluble in the solvent and less sticky
iii) - Cut out the yellow pigment
- put in organic solvent to dissolve the pigment
- filter and evaporate the filtrate to get the pigment
iv)Above the red pigment and below the edge.
b)-Heat the mixture aluminum chloride sublime and collect be cooler part of the tube and sodium chloride left at bottom of the tube
- Scratch the condense alcl3 place in a beaker
ii) Its most soluble in the solvent and less sticky
iii) - Cut out the yellow pigment
- put in organic solvent to dissolve the pigment
- filter and evaporate the filtrate to get the pigment
iv)Above the red pigment and below the edge.
b)-Heat the mixture aluminum chloride sublime and collect be cooler part of the tube and sodium chloride left at bottom of the tube
- Scratch the condense alcl3 place in a beaker

